Fujifilm's COVID-19 drug enters testing in US; will be free to 20 countries
Japan offers Fujifilm's COVID-19 drug, Avigan, free to 20 countries, as the treatment begins clinical trials in the US
The best camera deals, reviews, product advice, and unmissable photography news, direct to your inbox!
You are now subscribed
Your newsletter sign-up was successful
Clinical testing for Fujifilm's COVID-19 treatment drug, Avigan, has officially commenced in the United States, following the testing that began in Japan at the end of March. Further, the Japanese government has pledged that the drug will be made freely available to 20 countries to aid in the fight against the coronavirus pandemic.
Fujifilm announced today the initiation of phase II clinical trials in the US to evaluate the safety and efficacy of Avigan (which also goes by the generic name favipiravir). The trial will take place in collaboration with Brigham and Women’s Hospital, Massachusetts General Hospital, and the University of Massachusetts Medical School, and will involve some 50 patients infected with COVID-19.
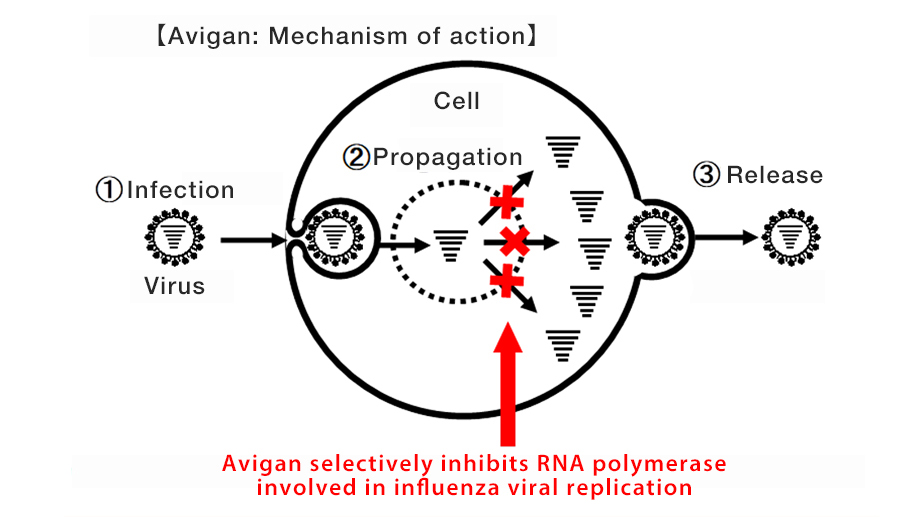
"Avigan, approved in Japan for manufacture and sale as an influenza antiviral drug, selectively inhibits RNA polymerase necessary for influenza virus replication," notes the announcement from Fujifilm.
"Due to this mechanism of action, it is expected that Avigan may potentially have an antiviral effect on the new coronavirus, because like influenza viruses, coronaviruses are single-stranded RNA viruses that also depend on viral RNA polymerase."
At the same time, the Japanese government will boost its stockpile of the drug, with Prime Minister Shinzo Abe pledging to triple the nation's supply in order to treat two million infected people.
“We will work with interested countries to expand clinical research on Avigan internationally,” Foreign Minister Toshimitsu Motegi told press on Tuesday.
According to Japan Times, "the 20 countries receiving the drug, which is currently undergoing clinical tests, include Bulgaria, the Czech Republic, Indonesia, Iran, Myanmar, Saudi Arabia and Turkey, with an additional 30 countries showing interest."
The best camera deals, reviews, product advice, and unmissable photography news, direct to your inbox!
The report notes that Japan is working directly with the United Nations, and will provide the UN Office for Project Services with a $1 million grant to buy and distribute the drug.
Read more:
Fujifilm has developed new coronavirus test that produces results in just 2 hours
Fujifilm's medical division successful in treating COVID-19
The best Fujifilm cameras in 2020: from X-mount mirrorless to GFX medium format

James has 25 years experience as a journalist, serving as the head of Digital Camera World for 7 of them. He started working in the photography industry in 2014, product testing and shooting ad campaigns for Olympus, as well as clients like Aston Martin Racing, Elinchrom and L'Oréal. An Olympus / OM System, Canon and Hasselblad shooter, he has a wealth of knowledge on cameras of all makes – and he loves instant cameras, too.
